by George Langford, Sc.D., Massachusetts Institute of Technology, Cambridge, MA, 1966
Copyright©2005 by George Langford
Cast Irons, High Alloy Steels, and Superalloys - Lesson 1 - Third specimen
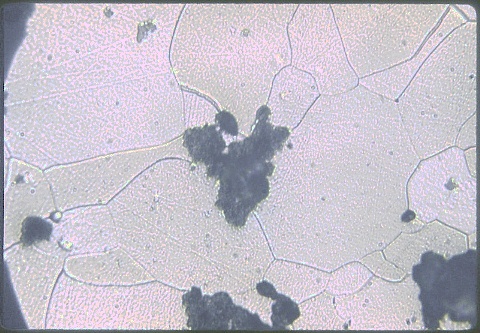
 This is a malleable cast iron after conversion to nodular
graphite, shown at 100X. This is a malleable cast iron after conversion to nodular
graphite, shown at 100X. The silicon content and size of the casting (i.e., the section thickness) were both kept sufficiently low that the nucleation of graphite was suppressed during solidification. The casting froze as a mixture of cementite plus austenite called white cast iron (from its characteristic fracture appearance, compared to graphitic grey cast iron). The room temperature microconstituent formed from the eutectic austenite plus iron carbide is called ledeburite, which is usually pearlite plus massive cementite. Upon prolonged heating at up to 930C over a period of several days, the cementite decomposes to austenite plus spheroidal graphite. During subsequent slow cooling to room temperature, the austenite decomposes to ferrite plus additional graphite. The silicon content is critical; too much causes graphite formation during freezing; too little prolongs the solid state graphitization process. About 1% silicon is best. |