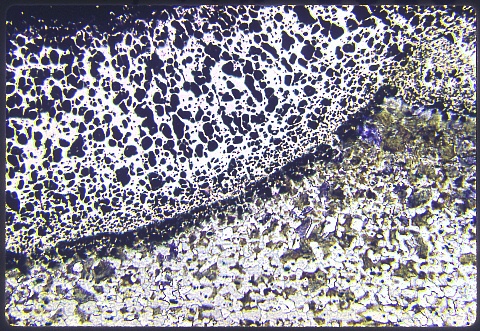
Silicon increases the chemical activity of carbon in iron, so the BCC alpha (ferrite) case at upper left in the photomicrograph has essentially no carbon left in it ... the carbon migrated inwards, carburizing the steel substrate. There was an interlayer of austenite ... which has now completely transformed to pearlite. Silicon slows down the austenite ==> ferrite plus cementite reaction much less than chromium does. That's porosity in the alpha (siliconized ferrite) case, which is a consequence of sulfur impurities in the steel.