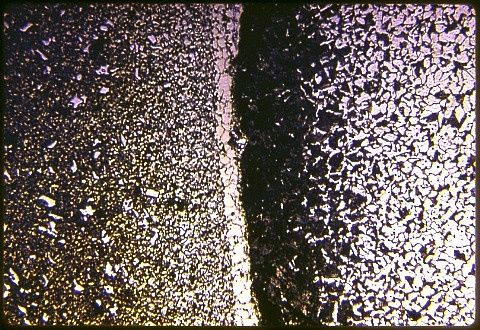
In the image at left, shown at 100X, the alloy rod is at left and the plain carbon matrix is at the right. There is no gap between the two, which were bonded together metallurgically during the hot working process by which the original assembly was reduced to the final diameter of about 0.5 inch.
Notice the duffusion zone - the alloy rod is locally depleted in carbon and the plain carbon matrix has become carburized. A layer of ferrite lies between.
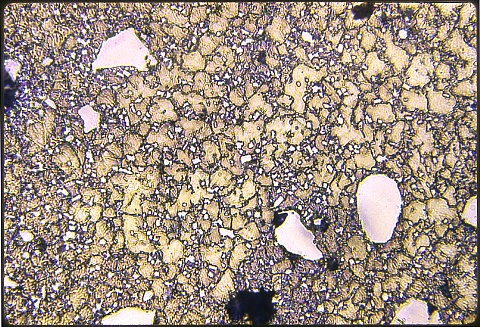
The image at left shows the alloy rod at 500X.
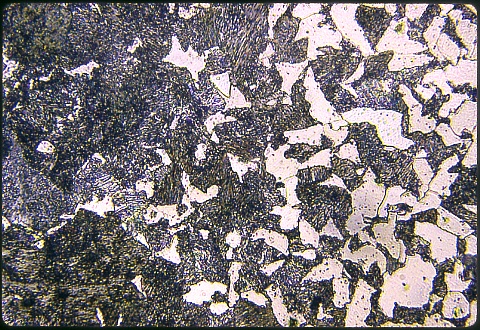
The frame below shows the plain carbon matrix at the same magnification; both were eteched at the same time with Nital.
Pause to formulate your response, then proceed to the answer below.