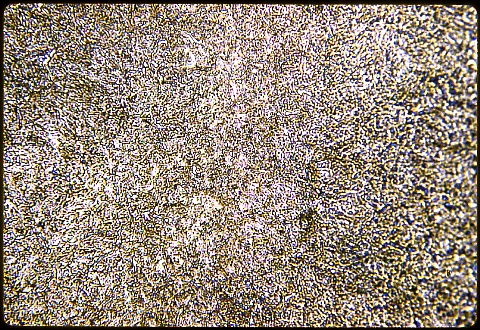
The three photomicrographs were all taken at 500X with a Nital etch. The first one, at left, shows the case. The second one shows the subsurface; and the third one shows the core. Be sure you understand the differences in case and core microstructures in these two specimens.
The surface case consists of martensite plus cementite without any retained austenite ... the austenite from which the martensite formed had a lower carbon content ... about 0.8% ... because the excess carbon was sequestered in the cementite formed just above the eutectoid temperature, even though the overall composition was hypereutectoid at the surface.

There is less pearlite here at a hypoeutectoid carbon level than in the specimen austenitized at 930C because of the higher carbon content of the austenite in equilibrium with ferrite. That is the converse of the hypereutectoid case. This austenite is now more hardenable because of its higher carbon content. That is why the patches of martensite could form next to the pearlite ... which did not completely transform from that austenite during the quench; it was this "retained" austenite from which the martensite formed.
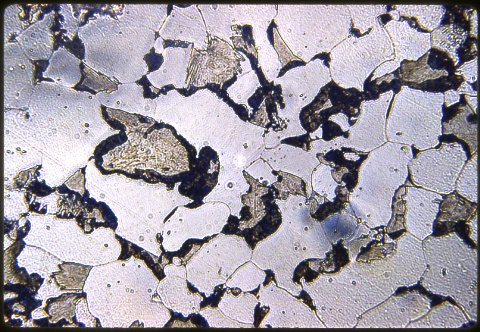
There is also some fine pearlite as well as a little more associated martensite than in the subsurface because of the faster quench rate of the overall specimen that begins, once the outer surface temperature drops below the boiling point of the water. The cooling rate during transformation of the subsurface austenite was actually slower because there was a steam envelope insulating the piece at the time that the austenite was decomposing there.