Microstructures
by
George
Langford, Sc.D., Massachusetts Institute of Technology, Cambridge, MA,
1966
Copyright©2005 by George Langford
Non Ferrous Alloys - Lesson 3 -
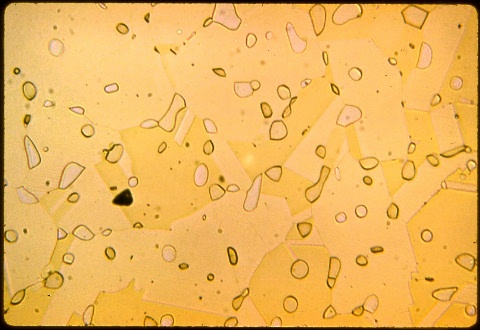
Sixth specimen
|
Beryllium copper ( copper with 2% beryllium and 1/4% cobalt
or nickel) is used to make non-sparking tools for use in environments
with severe explosion hazards. It must be carefully heat treated
to develop sufficient strength to perform like steel tools of the same
shape and size.
Remember - copper melts below 1100C whereas steels have melting points
around 1500C. Copper isn't as strongly bonded as iron and is
therefore generically weaker, given similar microstructures. This
photomicrograph is shown at 200X; the etchant was potassium chromate.
|

|
The copper - beryllium phase diagram has a monotectoid
reaction (gamma-1 going to alpha plus gamma-2) but this reaction is not
used for strengthening like the eutectoid reaction in the iron carbon
phase diagram. Instead, the alloy is solutionized in the
all-alpha region and is then quenched and precipitation hardened.
This specimen was wrought, solutionized at 775C and quenched.
It's rather soft in this condition, about Rockwell B55.
The microstructure consists of a sea of alpha grains (with annnealing
twins) and islands of beta (the dark yellow or whitish phase).
The beta probably forms because of the minor alloy additions and does
not participate during subsequent heat treatments.
Specimen 7 is next.
|

