
The hardness of the primary (white) phase was Rockwell C57; overall hardness was Rockwell C28. These are quite high for a non ferrous metal.
My magnifications are: 50X; 200X; 500X; and 1000X.
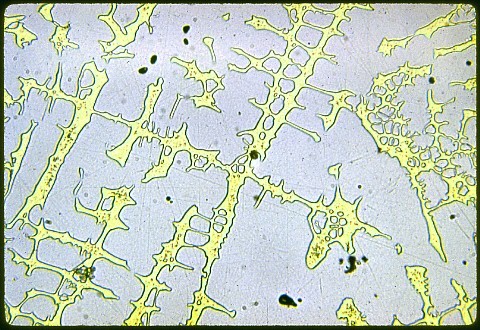
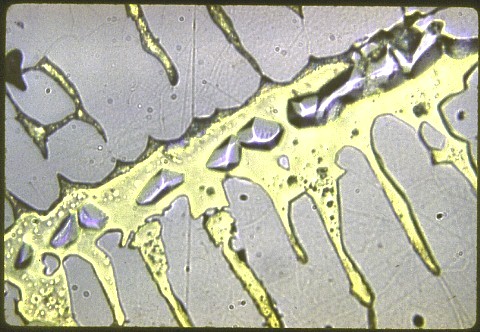
There wasn't much any of us could say about this one, but you're free to speculate ... and I'll cheerfully discuss it with you. All I can say is that there are two kinds of non-sparking alloys that are used for tools in flammable environments. One is berryllium copper (which was already discussed in an earlier lesson) and the other is aluminum bronze. But there's no aluminum here. Neither alloy has appreciable zinc, nickel, or iron. Hint: Look at the copper - iron and copper - nickel phase diagrams. Remember: The yellow phase isn't the hard one. It's just holding the white stuff together.
Write out your answer; complete all or part of the set; and then apply for a Username & Password so you can activate the link at the bottom of this page.