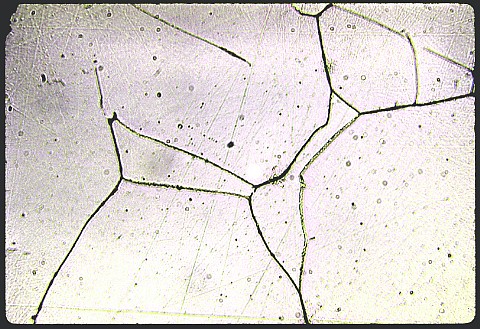
 The specimen at left is an
austenitic stainless steel
containing 18% chromium, 8% nickel, and a trace of almost unavoidable
carbon as an impurity. The magnification is 500X, and the
microstructure has been made visible by etching electrolytically in an
oxalic acid solution. Stainless steels are those that have more than 12% chromium,
which
permits a passive layer of either chromium oxide or adsorbed oxygen to
form, preventing access of further oxygen to the iron atoms. The
rate of corrosion in aqueous solutions and the rate of oxidation at
elevated temperatures in oxidizing atmospheres are thereby greatly
reduced. Since chromium stabilizes BCC ferrite, which has
marginal mechanical
properties, nickel is usually added to make FCC austenite the preferred
phase.
The specimen at left is an
austenitic stainless steel
containing 18% chromium, 8% nickel, and a trace of almost unavoidable
carbon as an impurity. The magnification is 500X, and the
microstructure has been made visible by etching electrolytically in an
oxalic acid solution. Stainless steels are those that have more than 12% chromium,
which
permits a passive layer of either chromium oxide or adsorbed oxygen to
form, preventing access of further oxygen to the iron atoms. The
rate of corrosion in aqueous solutions and the rate of oxidation at
elevated temperatures in oxidizing atmospheres are thereby greatly
reduced. Since chromium stabilizes BCC ferrite, which has
marginal mechanical
properties, nickel is usually added to make FCC austenite the preferred
phase. Inadvertent slow cooling after an annealing treatment permitted chromium carbide (Cr4C) to precipitate in this specimen on the austenite grain boundaries, where diffusion of carbon is very fast. The austenite next to these grain boundaries has been depleted of the chromium that went into the Cr4C, and so the metal next to those grain boundaries is no longer stainless. The etching effect seen above in the first photomicrograph is called grooving.
Specimen 3 is a failed stainless
steel.