
At 50X the cast, microsegregated, dendritic microstructure is evident.


Specimen 5 is a cast alpha brass.
 |
The six frames on this page describe a copper - 10% tin
bearing bronze containing added lead. The three photomicrographs
were made from essentially the same general area in a single
specimen. They look so different because of the vastly different
fields of view at the three magnifications. At 50X the cast, microsegregated, dendritic microstructure is evident. |
 |
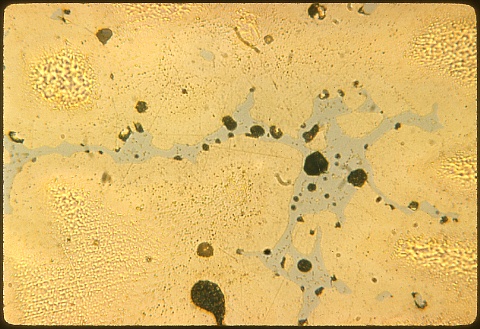
At 200X the bluish-white delta microconstituent can be
seen. The black areas in these two photomicrographs are either
shrinkage cavities or lead particles; can you tell one from the other
? |
 |
The coring that is evident above at 200X and at left at 500X
shows because the copper center (core) of each dendrite arm is
copper-rich and etches more slowly than does the outside of each
dendrite arm. Even the delta is cored, as you can see at left. |
 |
The copper - tin phase diagram should help you to interpret
this microstructure. There are several intermediate phases and
complex solid-state invariant reactions. |
 |
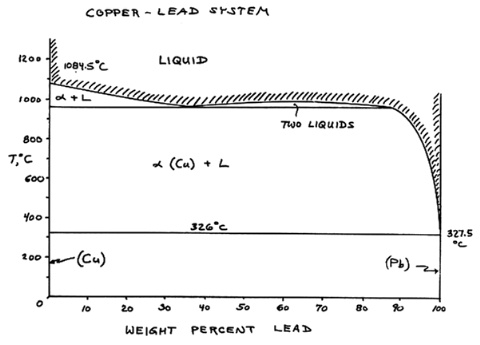
The copper - lead phase diagram shows that there is
essentially no solubility of lead in solid copper and that lead remains
molten in solidified copper above 326C. |
 |
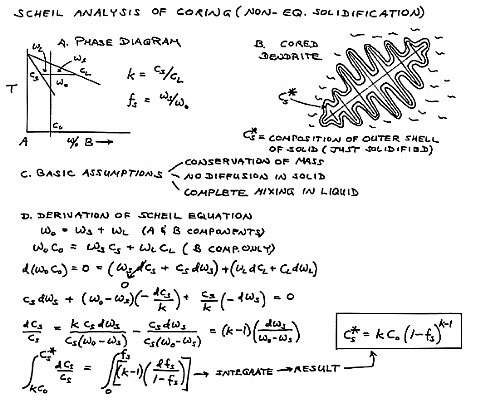
This is the formal development of the Scheil analysis of
coring (microsegregation) during solidification. The key
assumptions are that there is no diffusion in the solid and that there
is complete mixing in the liquid. The Scheil equation explains
why the delta phase appears in this alloy, even though alpha (copper
rich in tin) and lead should be the only microconstituents. The
last liquid to freeze is significantly enriched in tin. Specimen 5 is a cast alpha brass. |