

Specimen 6 is Muntz metal, an alloy with 40% zinc.
 |
This is a cast alpha brass at 50X. It has about 30%
zinc. Note the [very few] grain boundaries; these are much easier
to see than those in the preceding specimen.
There is brightness contrast because of the differring reflectivity of
the variously oriented dendrites. Each grain is a single,
separate dendrite with many closely spaced secondary arms. Note
the difference between the relatively long primary and short secondary
dendrite arms. There is coring in this specimen, too. |
 |
The copper - zinc equilibrium diagram says that the
beta phase is not stable at room temperature, even though it is
the normal microconstituent in higher zinc alloys. Beta phase is
not present in this specimen because zinc diffuses quite rapidly in
copper (compared to tin in copper, for example). Any beta phase
in the zinc-enriched last metal to freeze has dissolved into the alpha
phase. |
 |
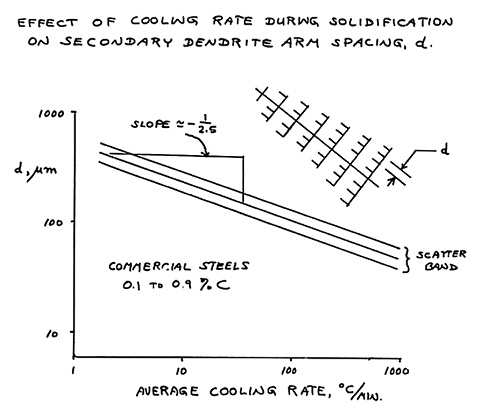
The dendrite arm spacing of a casting is indicative of the
cooling rate during freezing - coarse dendrites forming in large
castings and fine dendrites in small castings. Specimen 6 is Muntz metal, an alloy with 40% zinc. |