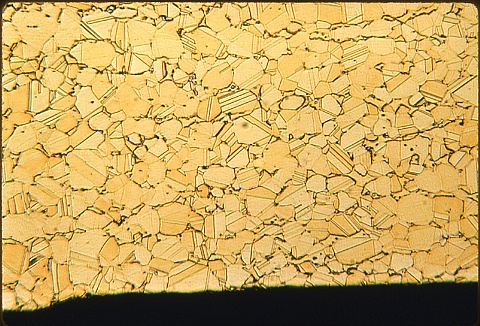
Look at all four photomicrographs before answering.
The upper pair show the outer edge at 100X ...

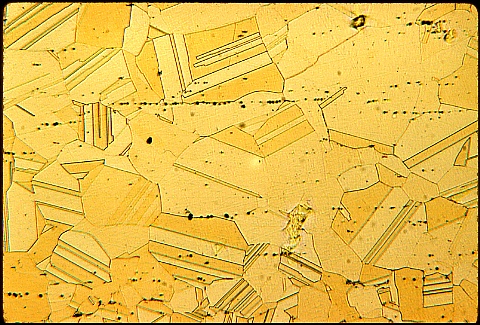
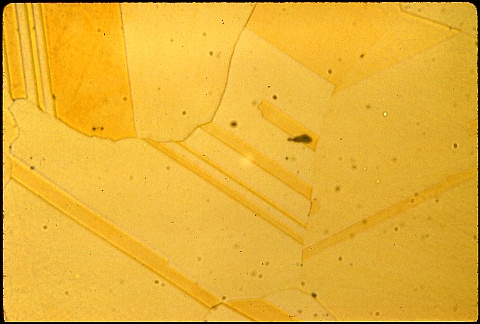
The manufacturer was "stuck" with many pieces which were too brittle to be used. He wanted to "rescue" them from the scrap barrel. What would you have done ?
Again, pause a while before looking at his solution.
