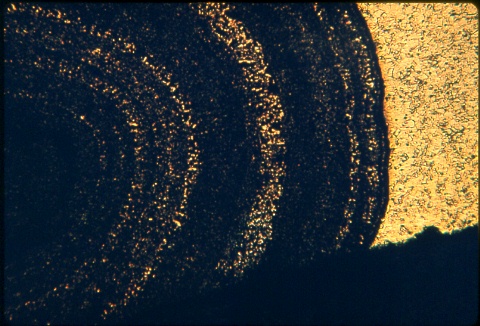
Ammonium hydroxide - hydrogen peroxide etchant.
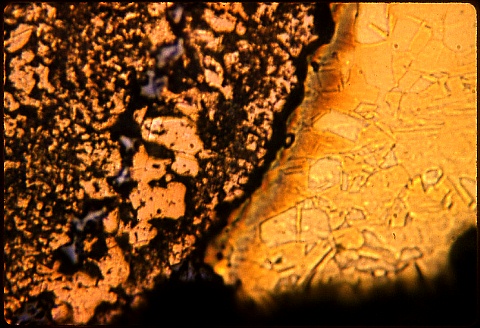
The water contained dissolved carbon dioxide and oxygen; the deposit on the inside of the pipe, which you cannot see here, was zinc carbonate.
What happened ?
Think a minute, then peek below.
| The next few
specimens are for your review of the microstructures you have seen so
far. They are all corrosion failures. |
 |
Yellow brass water pipe at 50X. Ammonium hydroxide - hydrogen peroxide etchant. |
 |
Here the magnification is 500X. The water contained dissolved carbon dioxide and oxygen; the deposit on the inside of the pipe, which you cannot see here, was zinc carbonate. What happened ? Think a minute, then peek below. |
| The pipe was dezincified - the
brass was dissolved and oxidized zinc was precipitated as zinc
carbonate, while the copper was redeposited as a spongy elemental
metalloic mass. What copper alloys are susceptible ? Think for a minute, then look. |
| Muntz metal (yellow brass)
especially, but in general, any brass with more than 15 weight percent
zinc. What alloys are immune ? Another pause, then proceed. |
| Red brass and
also bronzes with less than 15 percent
zinc.
Now go on to Specimen 12. |