|
I inadvertently made this unknown myself while trying to
make a rod of alpha - beta brass. I mixed up the right weight
percentages of copper and zinc, sealed them in vacuum in a Vycor tube,
and placed the tube in a furnace at a temperature high enough to melt
the alloy completely, yet low enough not to force the vapor pressure of
zinc above one atmosphere, lest the tube explode. My aim was to
get a 50:50 mixture of each phase, but every time I tried to take
the tube and its molten contents out of the furnace, the liquid would
start to boil vigorously because the cold tongs were making the
arrangement function like a heat pipe.
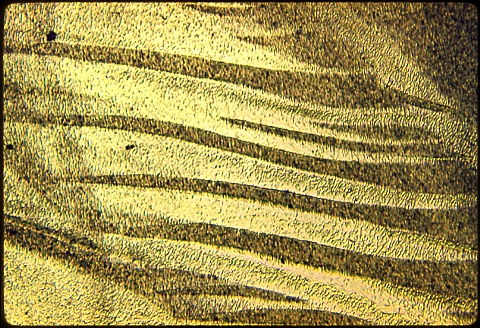
My first photomicrograph was made at 200X; then 500X and finally, 1000X.
|
|
The end result, after I gave up trying to avoid all this
alarming boiling and just took it out and stood it on end against the
leg of the table, was a rod that was 100% alpha at one end and 100%
beta at the other end. When I tried swaging (cold reducing the
diameter) the rod, this single grain of beta popped out. It was
about the size and shape of a beech nut.
See if you can figure out what this microstructure consists of, and
also how the rod ended up with such dramatic macrosegregation - it was
brown at one end and lemon yellow at the other end !
There were equal numbers of copper and zinc atoms.
|

|
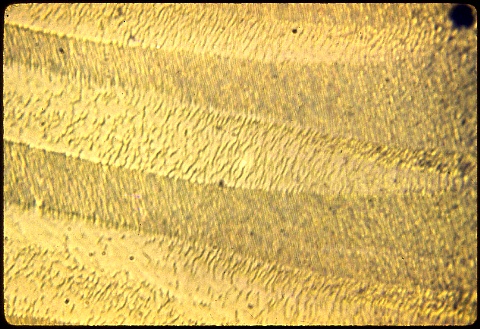
These are copper precipitates at 1000X.
Write
out your answer; complete all or
part of the set; and
then apply
for a
Username & Password so you can activate the link at the bottom of this page.
|