In Specimen 4 we go on to a three-component system.
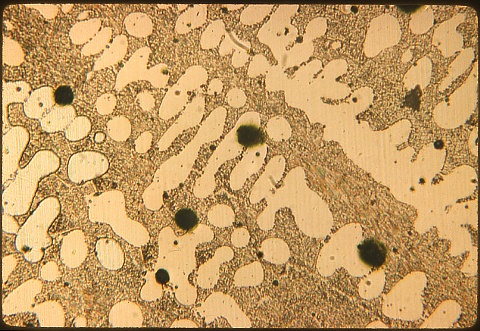
| This is underpoled copper, shown at 50X. The liquid
metal was stirred with a freshly cut sapling. No kidding; I saw
this done in about 1958 in a visit to a copper company. The
"sapling" was actually a tree trunk about forty feet long and six
inches in diameter, hung by the middle from a winch and manipulated by
three strong men. Hydrogen freed by heat from hydrocarbons in the
pole combines with dissolved oxygen in the molten copper to form steam
bubbles, deoxidizing the copper. Too little poling leads to
porosity due to steam evolution during freezing. This
photomicrograph shows a tremendous amount of eutectic compared to the
properly treated copper in Specimen 2 (previous). |
|
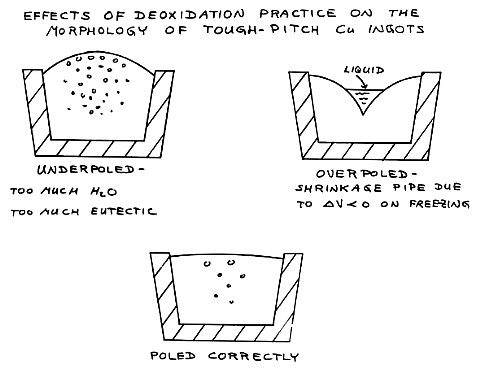
| This frame explains why the steam bubbles form in the
solidifying copper. |
|
 |
At 200X at left you can see the
classical eutectic microstructure of the metal. Compare to the divorced (phase-separated)
eutectic in
Specimen 2. |
 |
Poling is necessary to deoxidize
the copper. Too little poling
gives too much eutectic oxide and too much porosity as well as bulging
of the ingot. Too much poling permits too deep a shrinkage pipe
to form, leaving a severe depression in the center of the ingot which
leads to the formation of seams and cold shuts during subsequent hot
rolling. In Specimen 4 we go on to a three-component system. |